Interpreting Your COVID-19 Testing Results
Test Sensitivity Varies With Time Since Symptom Onset
Estimated Variation Over Time in Diagnostic Tests for Detection of COIVD-19 Infection Relative to Symptom Onset Estimated time intervals and rates of viral detection are based on data from several published reports. Because of variability in values among studies, estimated time intervals should be considered approximations and the probability of detection of COVID-19 infection is presented qualitatively. a Detection only occurs if patients are followed up proactively from the time of exposure. b More likely to register a negative than a positive result by PCR of a nasopharyngeal swab. From: Interpreting Diagnostic Tests for SARS-CoV-2. JAMA. Published online May 06, 2020. doi:10.1001/jama.2020.8259
Nasal Swab (RT-PCR)
The COVID-19 reverse transcriptase–polymerase chain reaction (RT-PCR) test identifies active viral infection using a nasal swab and detects active infection as well as asymptomatic carriers.
Quest Diagnostics performs the nasal swab RT-PCR. Quest reports that the concentration of SARS-CoV-2 RNA that was successfully detected with at least a 95% detection rate was calculated as 136 copies/mL. It is important to note that timing and adequate sampling determine this test's sensitivity. Recent data show that RNA positive rates peaked in upper respiratory tract specimens at 7–10 days after symptom onset and then steadily declined after that. Sensitivity also depends on an adequate sample being collected and the disease having advanced enough to have a higher number of viral RNA copies.
Antibody Tests
The COVID-19 antibody IgG enzyme-linked immunosorbent assays (ELISA) look for IgG antibodies in the blood to determine if your body has already responded to the novel coronavirus.
Roche Elecsys Anti-SARS-CoV-2 igG Total Antibody test. Roche reports an overall test specificity of 99.81 % (99.65 – 99.91 %) and a sensitivity in patients 14 days after PCR confirmation to be 100% (88.1 – 100 %). It is very important to note, that the sensitivity of the antibody tests is very dependent upon the timing of the test since symptom onset with the most sensitivity beginning 14 days after symptom onset.
Both, Quest Diagnostics and Roche offer testing in accordance with the FDA Emergency Use Authorization Guidance.
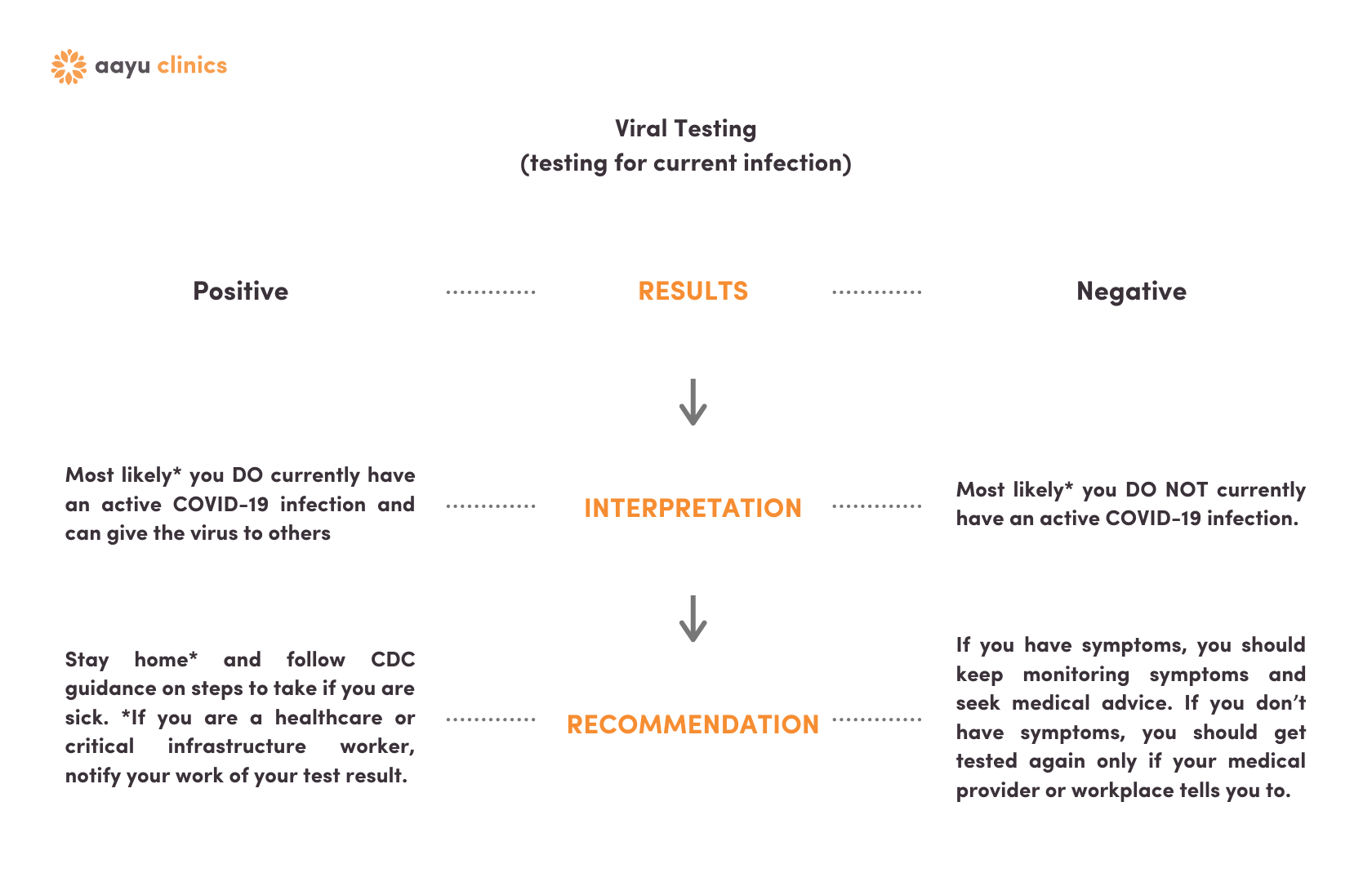
Viral Testing Results
Click here if you only had the nasal swab PCR test.
Viral & Antibody Results | Viral Positive
Click here if you had the nasal swab and the blood test and were viral positive.
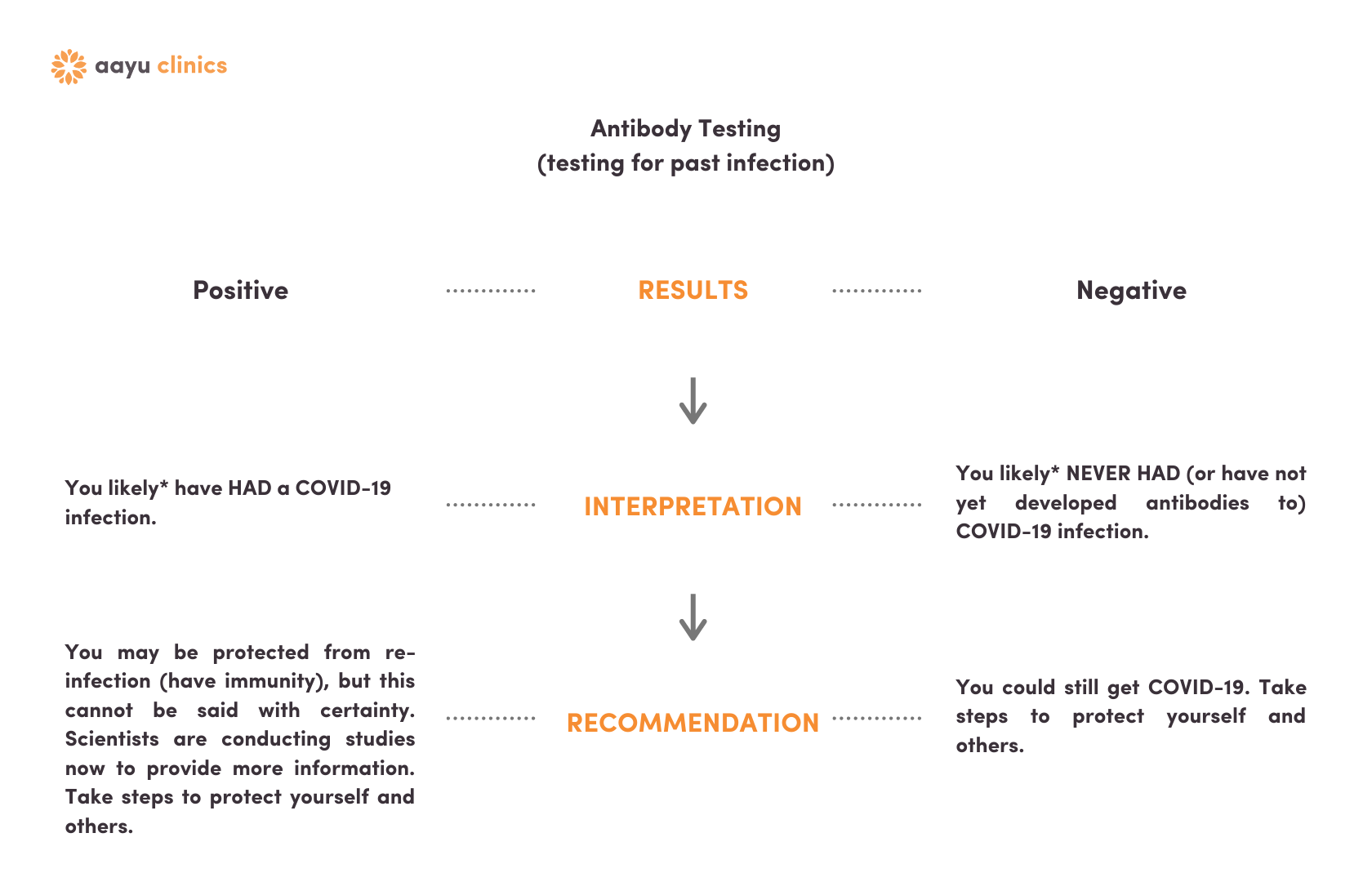
Antibody Testing Results
Click here if you only had the blood test.
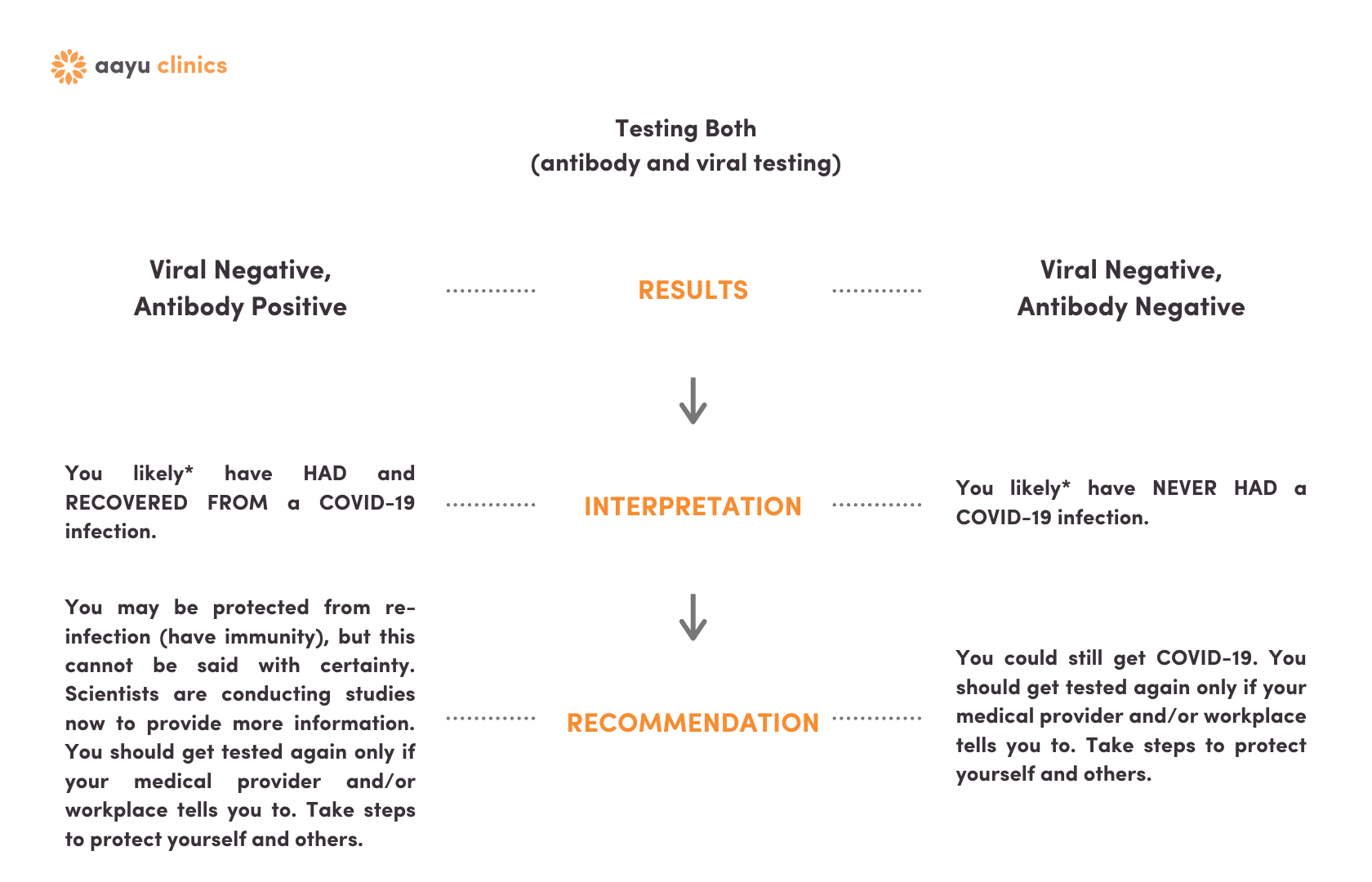
Viral & Antibody Results | Viral Negative
Click here if you had the nasal swab and the blood test and were viral negative.
What We Do Not Yet Know About Antibody Tests
Currently available commercial assays do not have titers, and without this information it is unclear how to identify “qualified” individuals for plasma donation
While extrapolation from other coronavirus infections allows us to be optimistic that detection of an IgG response will likely confer at least some protection to most people, we have no direct evidence of this for SARS-CoV-2.
Understanding which antibodies (if any) are protective is required for vaccine development. There are many different SARS CoV-2 IgG antibodies that may be produced, and each may have a different role. This should also be a consideration in assessing the clinical utility of tests designed to target specific antibodies.
Detection of Viral RNA by RT-PCR
Thus far, the most commonly used and reliable test for diagnosis of COVID-19 has been the RT-PCR test performed using nasopharyngeal swabs or other upper respiratory tract specimens, including throat swab or, more recently, saliva.
In most individuals with symptomatic COVID-19 infection, viral RNA in the nasopharyngeal swab becomes detectable as early as day 1 of symptoms and peaks within the first week of symptom onset. PCR positivity may persist beyond 3 weeks after illness onset when most mild cases will yield a negative result. However, a “positive” PCR result reflects only the detection of viral RNA and does not necessarily indicate presence of viable virus.
The sensitivity of this test is low in early illness, and is even lower in asymptomatic individuals, likely because of lower viral load, which means even more false negatives. The test's sensitivity is also dependent upon obtaining an adequate sample from inside the nose.
In some cases, viral RNA has been detected by RT-PCR even beyond week 6 following the first positive test. A few cases have also been reported positive after 2 consecutive negative PCR tests performed 24 hours apart. It is unclear if this is a testing error, reinfection, or reactivation.
In a recent study, attempts to isolate the virus in culture were not successful beyond day 8 of illness onset, which correlates with the decline of infectivity beyond the first week. That is in part why the “symptom-based strategy” of the Centers for Disease Control and Prevention (CDC) indicates that health care workers can return to work, if “at least 3 days (72 hours) have passed since recovery defined as resolution of fever without the use of fever-reducing medications and improvement in respiratory symptoms (e.g., cough, shortness of breath); and, at least 10 days have passed since symptoms first appeared. In patients with confirmed COVID-19 infection, RT-PCR positivity is higher in nasal swab specimens (63%), as compared to pharyngeal swab (32%) Specificity of most of the RT-PCR tests is 100% because the primer design is specific to the genome sequence of SARS-CoV-2. Occasional false-positive results may occur due to technical errors and reagent contamination.
Detection of Antibodies to SARS-CoV-2
COVID-19 infection can also be detected indirectly by measuring the host immune response to COVID-19 infection. Serological diagnosis is especially important for patients with mild to moderate illness who may present late, beyond the first 2 weeks of illness onset. Serological diagnosis also is becoming an important tool to understand the extent of COVID-19 in the community and to identify individuals who are immune and potentially “protected” from becoming infected.
The most sensitive and earliest serological marker is total antibodies, levels of which begin to increase from the second week of symptom onset. Although IgG antibodies been found to be positive even as early as 7-9 days after symptom onset, higher levels occur around the 14th day of illness.
Recent studies have shown that IgG seroconversion occurred in all patients at/after the 14th day of clinical illness onset and the IgG antibodies persist beyond 7 weeks.